
Myocardial Perfusion and Function assessed by a Gated Cardiolite® Study
In patients with known or suspected CAD . . .
The Signs May Be There.

*Based on a prospective study of 5,807 consecutive patients, designed to determine the incremental prognostic value of SPECT for the prediction of cardiac death and the implications for risk stratification in patients undergoing dual-isotope SPECT with either exercise or pharmacological stress and followed for 642 ± 226 days, with prognostic data available for 5,183 patients.4
†Based on a study of 1,230 consecutive patients undergoing adenosine stress dual-isotope SPECT. The evaluation of referral patterns to catheterization was based on 1,159 patients, but prognostic data presented here is based on 1,079 patients to (1) determine the incremental prognostic value of adenosine stress SPECT; (2) evaluate the ability of SPECT to successfully risk stratify patients in various clinical risk categories; and (3) assess the actual use of this test by healthcare professionals relative to goals 1 and 2 by examining the rates and predictors of referral to catheterization after adenosine stress.5
Diagnostic and prognostic information may help you optimize your patient management decisions5,6
An MPI with Cardiolite® and gated SPECT combines perfusion and function in a single test1
Perfusion
- Helps establish:
- Extent of disease (single vessel vs multivessel)2,4
- Severity of disease
- Degree of reversibility
- Predictor of MI and cardiac death4,5
The greater the perfusion defect, the greater the risk of MI or cardiac death4,5
Function
- Helps establish:
- LVEF and LV function2,3
- Wall motion
- Wall thickening
- Predictor of cardiac death3,7
The lower the ejection fraction, the greater the risk of cardiac death3,7
- Cardiolite® with gated SPECT provides incremental prognostic information over perfusion alone in patients with known or suspected coronary artery disease (CAD)3,7
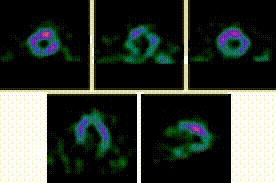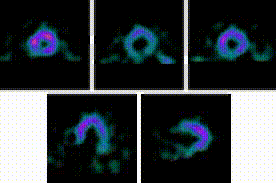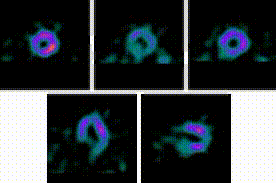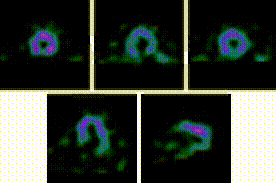
5-Fold Greater Predictability Over Clinical Testing or Clinical Testing Plus Exercise Treadmill Testing (ETT)6‡§
5-fold incremental prognostic value demonstrated in 2113 patients with no history of CAD6 |
 |
‡An MPI with Cardiolite® and gated SPECT6
§Based upon a study of 2,268 consecutive patients undergoing dual-isotope MPI and followed up for 566 ± 142 days, with 2,200 patients available for follow-up; however, the prognostic data was based on a population of 2,113 patients. This study was designed to determine the incremental prognostic value of exercise stress MPI in patients without previously defined CAD, to define the clinical role of the test in risk stratification in this population, and to determine the impact of this test on patient management as measured by post-nuclear testing referral of patients to catheterization and revascularization6
Rely on the Power of Information Available to You
It All Adds Up to Information to Help Make Confident Patient Management Decisions
- Cardiolite® [package insert]. N. Billerica, MA: Lantheus Medical Imaging.
- Dorbala S, Ananthasubramanium K, Armstrong IS, et al. ASNC Imaging Guidelines: Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing and interpretation. J Nucl Cardiol. 2018;25:1784-1846.
- Sharir T, Germano G, Kang X, et al. Prediction of myocardial infarction versus cardiac death by gated myocardial perfusion SPECT: risk stratification by the amount of stress-induced ischemia and the poststress ejection fraction. J Nucl Med. 2001;42:831-837.
- Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543.
- Hachamovitch R, Berman DS, Kiat H, et al. Incremental prognostic value of adenosine stress myocardial perfusion single-photon emission computed tomography and impact on subsequent management in patients with or suspected of having myocardial ischemia. Am J Cardiol. 1997;80:426-433.
- Hachamovitch R, Berman DS, Kiat H, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93:905–914.
-
Sharir T, Germano G, Kavanaugh PB, et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion SPECT. Circulation. 1999;100:1035-1042.


