
Risk Stratification
An MPI With Cardiolite® Gives You the Information to Help Manage Your Patients.1
Annual rates of cardiac death and MI by scan result2* |
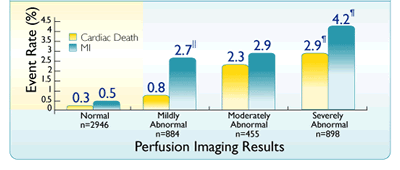 |
*Based on a prospective study of 5,807 consecutive patients, designed to determine the incremental prognostic value of SPECT for the prediction of cardiac death and the implications for risk stratification in patients undergoing dual-isotope SPECT with either exercise or pharmacological stress and followed for 642 ± 226 days, with prognostic data available for 5,183 patients.2
IIStatistically significant (P<0.05) increase in rate of MI versus cardiac death within scan category2
¶Statistically significant increase as a scan result2
Adapted from Hachamovitch R et al.2
The Signs May Be There in Your Patients With Diabetes and Known or Suspected CAD§
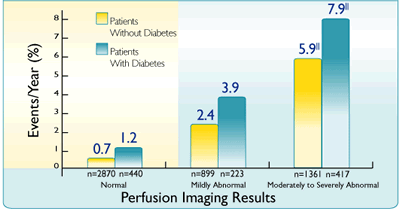
Annual rate of hard events (cardiac death and MI) as a function of scan result in patients with diabetes and known or suspected CAD3¶ |
 |
§The indications for Cardiolite® are not specific to patients with diabetes. Cardiolite® was shown to be an effective prognostic tool in the evaluation of patients with known or suspected CAD. This was determined in 3 pivotal trials enrolling 1,596 patients, including 311 patients with diabetes, which were used as the basis for approval.4
||Statistically significant (P<0.001) increase in event rates as a function of summed stress score3
Adapted from Kang X et al.3
In patients with diabetes, and with stable CAD and a normal Cardiolite® scan, data indicate a 1–2% risk of cardiac death or MI in the following year3¶#
¶Based on a prospective study of 1,271 consecutively registered patients with diabetes and 5,862 patients without diabetes with known or suspected CAD designed to evaluate the incremental value of stress (exercise and pharmacologic) myocardial perfusion SPECT in patients with diabetes.3
#A second prospective study of 929 patients with diabetes and 3,826 patients without diabetes with symptoms of CAD. 4,755 patients were followed for 2.5 (+1.5) years for subsequent occurrence of cardiac death, MI, or revascularization. A separate survival analysis was performed comparing patients with and without diabetes who had normal stress MPI images. For the first 2 years of the study follow-up, patients with normal stress MPI images had similar survival curves, irrespective of their diabetic status.5
Rely on the Information of an MPI With Cardiolite® for Risk Stratification of Your Patients
SCAN RESULTS (at stress) |
Annualized Risk of Cardiac Events |
Potential Treatment Implications ** |
 |
<1% risk of both MI and cardiac death2,6 | Risk factor modification in addition to current regimen6 |
 |
Low risk of cardiac death; intermediate risk of MI2,6 |
|
 |
Intermediate-to-high risk of both MI and cardiac death2,6 |
|
Scans contributed by Howard Lewin, MD, of Cardiac Imaging Associations
**Results from a gated MPI with Cardiolite® along with other clinical evaluations and test results should all be considered in making patient management decisions.
Is the predicted cardiac mortality in a nuclear stress test with pharmacologic stress the same in women and men?
Predicted cardiac death in women and men as a function of scan result when pharmacologic stress is used7††, 8††† |
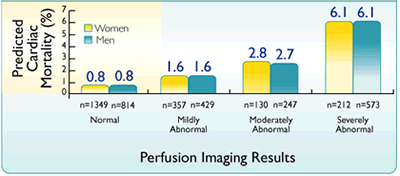 |
††In a study population that consisted of 6,173 consecutive patients with known or suspected CAD who underwent dual isotope MPI with pharmacological stress and followed up for 27 ± 8.8 months ; the final population included 2,677 males and 2,656 females7
Adapted from Berman DS et al.7
†††In a second prospective study of 2,377 consecutive patients (1,226 men and 1,151 women) who underwent exercise or dypyridamole Tc-99m sestamibi SPECT and who were followed up over 15± 8 months where only 2,228 patients were available for follow-up; to evalutate gender differences in the use of Tc-99m sestamibi for subsequent referrals for invasive procedures and its prognostic value in predicitng subsequent cardiac events.8
In women and men, a gated nuclear stress test with Cardiolite®:
- Adds incremental value in the prediction of cardiac death7
- Provides information that may assist you in making patient management decisions1
- Cardiolite® [package insert]. N. Billerica, MA: Lantheus Medical Imaging.
- Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543.
- Kang X, Berman DS, Lewin HC, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography in patients with diabetes mellitus. Am Heart J. 1999;138:1025–1032.
- Data on file. Lantheus Medical Imaging, Inc.
- Giri S, Shaw LJ, Murthy DR, et al. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation. 2002;105:32–40.
- Hachamovitch R. Risk assessment of patients with known or suspected CAD using stress myocardial perfusion SPECT: Part II: determining cost-effective test strategies. Rev Cardiovasc Med. 2001;2:41–47.
- Berman DS, Kang X, Hayes SW, et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared with men: impact of diabetes mellitus on incremental prognostic value and effect on patient management. J Am Coll Cardiol. 2003;41:1125–1133.
- Travin MI, Duca MD, Kline GM, et al. Relation of gender to physician use of test results and to the prognostic value of stress technetium 99m sestamibi myocardial single-photon emission computed tomography. Am Heart J. 1997;134:73-82.


